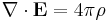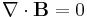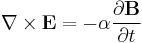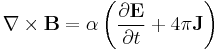Atomic units
Atomic units (au or a.u.) form a system of units convenient for atomic physics, electromagnetism, and quantum electrodynamics, especially when the focus is on the properties of electrons. There are two different kinds of atomic units, which one might name Hartree atomic units and Rydberg atomic units, which differ in the choice of the unit of mass and charge. This article deals with Hartree atomic units. In au, the numerical values of the following four fundamental physical constants are all unity by definition:
Atomic units are often abbreviated "a.u." or "au". Confusingly, this is the same abbreviation as Astronomical Units, Arbitrary Units, and Absorbance Units.
Contents |
Use and notation
Atomic units, like SI units, have a unit of mass, a unit of length, and so on. However, the use and notation is somewhat different from SI.
For example, the unit of mass in atomic units is the electron mass, usually denoted 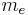 and with an SI value of about 9.1×10−31 kg. Suppose some particle has a mass m which is 3.4 times the electron mass. In atomic units, the value of m can be written three ways:
and with an SI value of about 9.1×10−31 kg. Suppose some particle has a mass m which is 3.4 times the electron mass. In atomic units, the value of m can be written three ways:
- "m=3.4me". This is the clearest notation (but least common), where the atomic unit is included explicitly as a symbol.[1]
- "m=3.4 a.u." ("a.u." means "expressed in atomic units"). This notation is ambiguous: Here, it means that the mass m is 3.4 times the atomic unit of mass. But if a length L were 3.4 times the atomic unit of length, the equation would look the same, "L=3.4 a.u." The dimension needs to be inferred from context.[1]
- "m=3.4". This notation is similar to the previous one, and has the same dimensional ambiguity. It comes from formally setting the atomic units to 1, in this case me=1, so 3.4me=3.4.[2][3]
Fundamental atomic units
| Fundamental Atomic Units | ||||
|---|---|---|---|---|
| Dimension | Name | Symbol | Value in SI units | |
| mass | electron rest mass |  |
9.1093826(16)×10−31 kg | |
| charge | elementary charge | 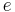 |
1.60217653(14)×10−19 C | |
| angular momentum | Reduced Planck's constant | 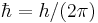 |
1.05457168(18)×10−34 J·s | |
| electric constant | Coulomb force constant | 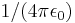 |
8.9875517873681×109 kg·m3·s-2·C-2 | |
Derived atomic units
Below are given a few derived units. Some of them have proper names and symbols assigned, as indicated in the table. Most symbols are defined in this table or the table above, but also α is the fine-structure constant, ε0 is the permittivity of vacuum, c is the vacuum speed of light, and kB is Boltzmann constant.
| Derived Atomic Units | ||||
|---|---|---|---|---|
| Dimension | Name | Symbol | Expression | Value in SI units |
| length | Bohr radius | 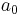 |
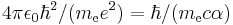 |
5.291772108(18)×10−11 m |
| energy | Hartree energy | 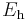 |
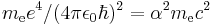 |
4.35974417(75)×10−18 J = 27.211 eV |
| time | 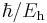 |
2.418884326505(16)×10−17 s | ||
| velocity | 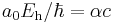 |
2.1876912633(73)×106 m·s−1 | ||
| force | 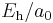 |
8.2387225(14)×10−8 N | ||
| temperature | 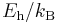 |
3.1577464(55)×105 K | ||
| pressure | 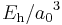 |
2.9421912(19)×1013 Pa | ||
| electric field | 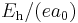 |
5.142×1011 V·m−1 | ||
There are two common variants of atomic units, one where they are used in conjunction with SI units for electromagnetism, and one where they are used with Gaussian-cgs units.[4] Although the units written above are the same either way (including the unit for electric field), the units related to magnetism are not. In the SI system, the atomic unit for magnetic field is
- 1 a.u. =
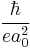 = 2.35×105 T = 2.35×109 G,
= 2.35×105 T = 2.35×109 G,
and in the Gaussian-cgs unit system, the atomic unit for magnetic field is
- 1 a.u. =
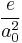 = 1.72×103 T = 1.72×107 G.
= 1.72×103 T = 1.72×107 G.
(These differ by a factor of α.)
Other magnetism-related quantities are also different in the two systems. An important example is the Bohr magneton: In SI-based atomic units,[5]
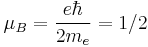 a.u.
a.u.
and in Gaussian-based atomic units,[6]
 a.u.
a.u.
Bohr model simplified
Atomic units are chosen to reflect the properties of electrons in atoms. This is particularly clear from the classical Bohr model of the hydrogen atom in its ground state. The ground state electron orbiting the hydrogen nucleus has (in the classical Bohr model):
- Orbital velocity = 1
- Orbital radius = 1
- Angular momentum = 1
- Orbital period = 2π
- Ionization energy = 1⁄2
- Electric field (due to nucleus) = 1
- Electrical attractive force (due to nucleus) = 1
Comparison with Planck units
Both Planck units and au are derived from certain fundamental properties of the physical world, and are free of anthropocentric considerations. It should be kept in mind that au were designed for atomic-scale calculations in the present-day universe, while Planck units are more suitable for quantum gravity and early-universe cosmology. Both au and Planck units normalize the reduced Planck constant. Beyond this, Planck units normalize to 1 the two fundamental constants of general relativity and cosmology: the gravitational constant G and the speed of light in a vacuum, c. Atomic units, by contrast, normalize to 1 the mass and charge of the electron, and a0, the Bohr radius of the hydrogen atom.
Let α denote the fine structure constant, α ≈ 1/137.036. While the speed of light is 1 in Planck units, it is 1/α ≈ 137.036 in au. The orbital velocity of an electron around a small atom is around 1 in atomic units, so the discrepancy between the velocity units in the two systems reflects the fact that electrons orbit small atoms much slower than the speed of light (around 2 orders of magnitude slower).
There are much larger discrepancies in some other units. For example, the unit of mass in atomic units is the mass of an electron, while the unit of mass in Planck units is the Planck mass, a mass so large that if a single particle had that much mass it might collapse into a black hole. Indeed, the Planck unit of mass is 22 orders of magnitude larger than the au unit of mass. Similarly, there are many orders of magnitude separating the Planck units of energy and length from the corresponding atomic units.
Quantum mechanics and electrodynamics simplified
The (non-relativistic) Schrödinger equation for an electron in SI units is
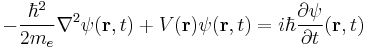 .
.
The same equation in au is
 .
.
For the special case of the electron around a hydrogen atom, the Hamiltonian in SI units is:
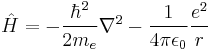 ,
,
while atomic units transform the preceding equation into
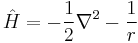 .
.
Finally, Maxwell's equations take the following elegant form in au:
Extending the atomic units to electromagnetism, there is some ambiguity in defining the atomic unit of magnetic field, electric current, etc. The above Maxwell equations use the cgs-Gaussian unit convention, in which a plane wave has electric and magnetic fields of equal magnitude.
See also
- Planck units
- Natural units
- Various extensions of the CGS system to electromagnetism.
References
- ↑ 1.0 1.1 Elementary quantum chemistry by Frank L. Pilar p155
- ↑ Group theory and chemistry by David M. Bishop, p217
- ↑ Springer handbook of atomic, molecular, and optical physics, by Gordon W. F. Drake, page 5
- ↑ Lecture notes from the University of Colorado
- ↑ Lecture notes from Universitatea Babes-Bolyai
- ↑ Atomic physics: an exploration through problems and solutions by Budker et al., page 380
- H. Shull, G.G. Hall (1959). "Atomic Units". Nature 184 (4698): 1559. doi:10.1038/1841559a0.
- G.W.F. Drake (ed.) (2006). Springer Handbook of Atomic, Molecular, and Optical Physics (2nd ed.). Springer. ISBN 978-0-387-20802-2.